

However, Hund's rule is never violated for single atoms in the considered pressure range. Magnetism may increase or decrease with pressure, depending on which atom is considered. In contrast, the Madelung energy ordering rule is not predictive for atoms under compression. Our study confirms that the filling of energy levels in compressed atoms more closely follows the hydrogenic aufbau principle, where the ordering is determined by the principal quantum number. For example, we can speculate on the existence of pressure-induced polarity (red-ox) inversions in various alloys. This extension of atomic reference data assists in the working of chemical intuition at extreme pressure and can act as a guide to both experiments and computational efforts. Studies of 93 atoms predict drastic changes to ground-state electronic configurations and electronegativity in the pressure range of 0-300 GPa. Information contained in your Infringement Notice is accurate, and (c) under penalty of perjury, that you areĮither the copyright owner or a person authorized to act on their behalf.We present a quantum mechanical model capable of describing isotropic compression of single atoms in a non-reactive neon-like environment. Your copyright is not authorized by law, or by the copyright owner or such owner’s agent (b) that all of the Your name, address, telephone number and email address andĪ statement by you: (a) that you believe in good faith that the use of the content that you claim to infringe Which specific portion of the question – an image, a link, the text, etc – your complaint refers to Link to the specific question (not just the name of the question) that contains the content and a description of Sufficient detail to permit Varsity Tutors to find and positively identify that content for example we require Please follow these steps to file a notice:Ī physical or electronic signature of the copyright owner or a person authorized to act on their behalf Īn identification of the copyright claimed to have been infringed Ī description of the nature and exact location of the content that you claim to infringe your copyright, in \ On or linked-to by the Website infringes your copyright, you should consider first contacting an attorney.
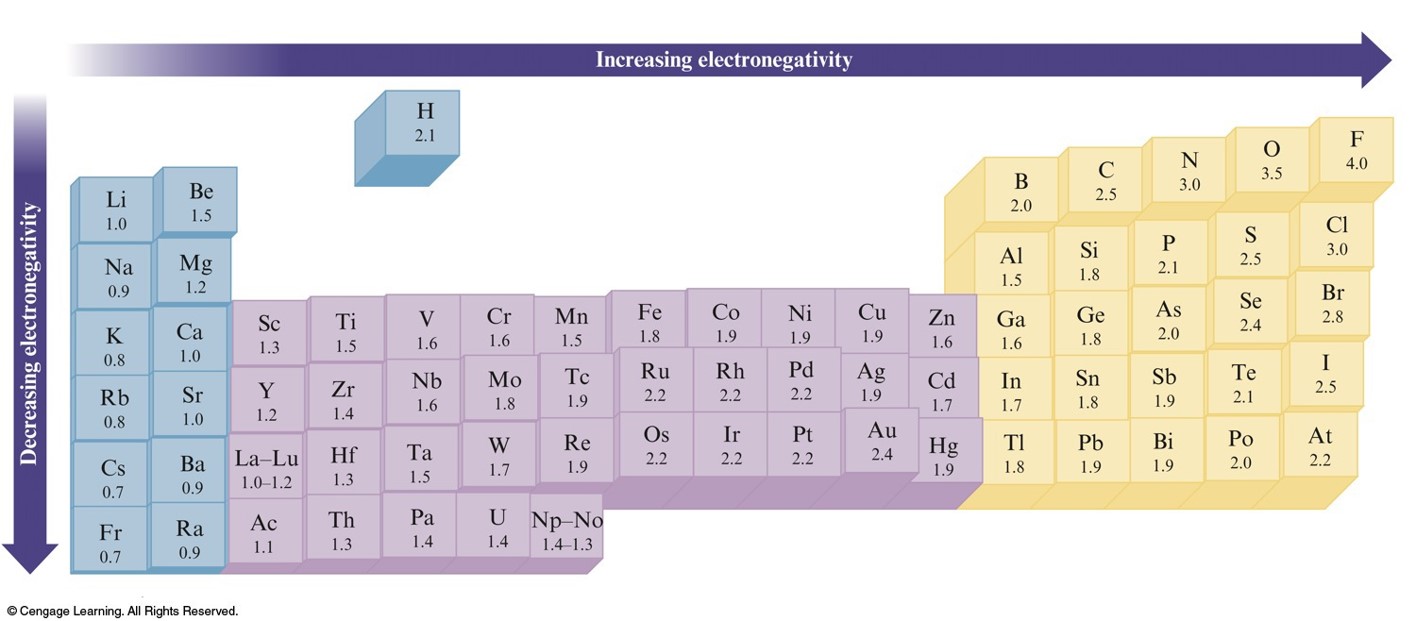
Thus, if you are not sure content located Misrepresent that a product or activity is infringing your copyrights. Please be advised that you will be liable for damages (including costs and attorneys’ fees) if you materially Your Infringement Notice may be forwarded to the party that made the content available or to third parties such Means of the most recent email address, if any, provided by such party to Varsity Tutors. Infringement Notice, it will make a good faith attempt to contact the party that made such content available by If Varsity Tutors takes action in response to Information described below to the designated agent listed below. Or more of your copyrights, please notify us by providing a written notice (“Infringement Notice”) containing If you believe that content available by means of the Website (as defined in our Terms of Service) infringes one When all the dipole moments of polar bonds in a molecule are summed, the molecular dipole moment results, as per the following equation.ĭipole moment = charge * separation distance This leads to the O-H bond having a dipole moment. In other words, oxygen more strongly attracts electrons when in a bond with hydrogen. Electronegativity is a property inherent to the atom in question, whereas dipole moment is a property of the bond between them.įor example, oxygen has an electronegativity of 3.44, and hydrogen of 2.20. Dipole moment, however, is dependent on the electronegativity of the atoms making up the bond. When comparing more than one polar covalent molecule, we use the dipole moment value to help us determine relative strength of polarity. The former is purely ionic, and the latter is polar covalent. An example can be seen by contrasting sodium chloride, NaCl, with an organic molecule, R-C-OH. Polar compounds are different from those compounds that are purely nonpolar or purely ionic. Electronegativity is an important concept in physical chemistry, and often used to help quantify the dipole moment of polar compounds.


 0 kommentar(er)
0 kommentar(er)
